A major challenge in developing viral-based therapies, such as gene therapies, CAR-T cell immunotherapy, and oncolytic virotherapy, is that the most efficient vectors and viruses are not specific, preventing their direct administration to the patient. Prof. Rubinstein and his team developed adapter molecules capable of directing viral vectors to specific target cells, ex vivo and in vivo. This technology can be applied to develop oncolytic viruses, in-vivo gene therapies, and generation of CAR-T cells in vivo (inside the patient), thus significantly reducing the time, complexity, and cost of these treatments.
VSV-G-pseudotyped lentiviral vectors (VSV-G-LVV) are the most commonly used tools for inserting genetic elements ex vivo into eukaryotic cells for gene therapy and CAR-T cell therapy. These therapies are complex and very expensive, and hence of limited use. Administration of these vectors directly to the patients will simplify these procedures and will greatly reduce their costs. These vectors use the low-density lipoprotein receptor (LDLR) for cell entry. Since LDLR is expressed in most cell types, directing VSV-G-LVV to specific cell types in vivo for gene therapy and CAR-T cell therapy is a major challenge. Oncolytic virotherapy by VSV is similarly hampered by the same non-specific cell targeting.
Prof. Rubinstein and his team developed adapter molecules that specifically bind and modify the target specificity of VSV and VSV-G-LVV. Their oncolytic potency and high transduction efficiency are maintained and are now combined with tailored specific cell targeting that will allow their direct administration to patients, thereby reducing the complexity of these procedures and their cost.
These adapters are fusion proteins comprised of two covalently bonded components: an anchoring component that binds to VSV and VSV-G-LVV, and a targeting moiety, which binds to a uniquely expressed receptor on the target cells. The adapters are produced in vitro and used to coat the vectors. The coated vectors may now be administered directly to the patient – for oncolytic virotherapy, gene therapy, and CAR-T cell therapy, thus reducing the complexity and costs of these treatments.
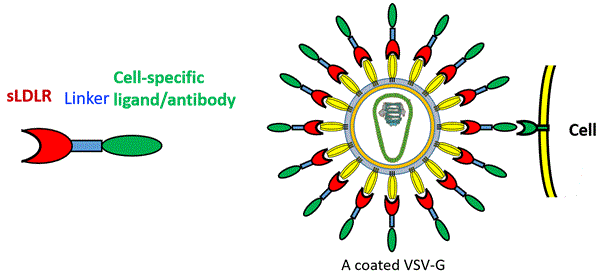
- Specific and efficient oncolytic virotherapy
- In vivo-generation of CAR-T cells – enabling significantly faster and cheaper treatment
- In-vivo targeted gene therapy for monogenic disorders, e.g., cystic fibrosis (CF). For CF, the adapter will allow gene therapy by aerosol-inhaled VSV-G-LVV that encodes CFTR.
The team synthesized and produced several different adapters for coating VSV and VSV-G-LVV and tested their efficacy and specificity on the relevant cell types. One adapter, sLDLR-CEACAM8, allowed specific binding of VSV-G-LVV to lung endothelial cells through their apical (air) side. e.g., for cystic fibrosis. It also directed VSV to colon and pancreatic cancer cells. Another adapter, sLDLR-DARPin56F3, directed VSV-G-LVV toward CD8+ T cells for generating CAR-T cells in vivo.


