Alzheimer's Disease (AD) is an age-associated neurodegenerative disorder characterized clinically by a decline in cognitive function. It is the most common type of dementia, with 5.8 million Americans living with AS in 2020. There are currently no curative or preventive treatments for the disease, which drastically affects patients' quality of life and impses a tremendous burden on their family and caregivers. Therefore, there is a substantial clinical and environmental need for curative therapies for AD. The group of Prof. Ruth Arnon developed a treatment for AD that prevents the pathological manifestation of the disease and improved cognitive function in an AD mice model.
Alzheimer's disease (AD) is an age-associated, irreversible neurodegenerative disorder. AD is characterized by the death of brain cells, which leads to a progressive decline in memory and cognitive abilities, including thinking, language, and learning. AD is the most common cause of dementia and the sixth leading cause of death in the United States1.
There are currently only six available treatments for AD. However, they all provide only symptomatic relief and will not cure the disease or prevent it from worsening over time. In light of the absence of disease-modifying treatments (DMTs), there is a substantial clinical and environmental need for curative therapies for AD.
Prof Ruth Arnon and her team developed a treatment for AD based on a mixture of two peptides that prevent plaque formation and cognitive deficiency.
The hallmark pathologies of Alzheimer's disease are the extracellular beta-amyloid (Aß) plaques and intracellular neurofibrillary tangles (NFTs), which are accompanied by the death of neurons and damage to brain tissue. The Aß plaques contain fragments of 40 or 42 amino acid peptides produced by proteolytic cleavage of the amyloid precursor protein (APP), while NFTs are composed of hyper-phosphorylated Tau protein. The causes for these plaque formations are not well understood yet but are suspected to result from proteins' misfolding and aggregation processes. It is currently unclear which of these two proteinaceous aggregates causes the disease. Still, disappointing results of single-target therapies suggest that dual amyloid and Tau targeting approaches should be considered.
The team led by Prof. Ruth Arnon demonstrated that purified APP and Tau proteins bind each other in vitro. They identified the peptide sequences in each protein that mediated this interaction and showed that their synthetic versions could form a complex. They identified two peptides (one originates for APP and one from Tau) that inhibit the binding of APP to Tau in vitro. Finally, the team showed that nasal administration of these peptides mixture to AD mice model reduced plaque formation, decreased soluble Aß levels in the brain, and improved the animals' cognitive function (Figure 1). The treatment prevented the deterioration of cognitive functions when initiated at an early stage before cognitive deficiency was evident or at later stages after symptoms initiation, leading to a full regain of cognitive function.2
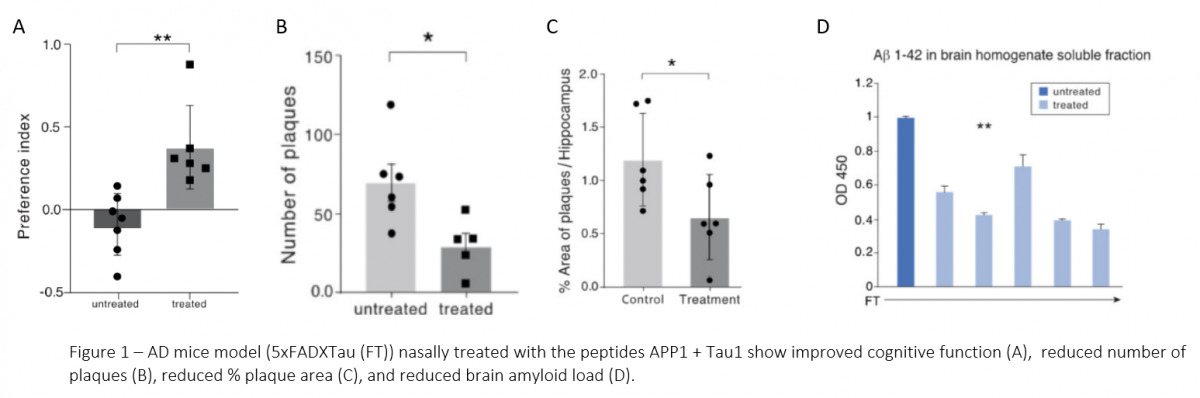
- Treatment for AD that directly affects the pathophysiology of the disease and prevents its progression
The team proved that APP and Tau bind in vitro, identified peptides that prevented this interaction, and demonstrated the effectiveness of a mixture of these peptides in an AD mice model.
References
2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020;16(3):391-460. doi:10.1002/alz.12068
Maron R, Armony G, Tsoory M, Wilchek M, Frenkel D, Arnon R. Peptide Interference with APP and Tau Association: Relevance to Alzheimer's Disease Amelioration. Int J Mol Sci. 2020;21(9):3270. doi:10.3390/ijms21093270


