Traceless covalent modification of proteins, including joining peptides or adding chemical groups, is extremely useful for numerous applications in chemical biology, protein chemistry, and biotechnology. A powerful tool currently used rely on Inteins, protein domains that auto-catalyze peptide bond formation. However, since inteins contain cysteine in their catalytic site, the reaction can only be made under reducing conditions, thus limiting the scope of proteins that can be modified. Prof. Pietrokovski and his collaborators at the University of Münster developed cysteine-less inteins that efficiently modify proteins under native and physiological conditions. They demonstrated their use with a full-length IgG antibody, an Fc fragment of an IgG, and nanobodies.
Modifying or joining proteins with other proteins/ peptides/chemical groups in a traceless manner is extremely useful for numerous applications. Existing solutions rely on Inteins, which are protein domains that auto-catalyze proteins’ posttranslational splicing reactions. Although this is a powerful tool that can be used both in vivo and in vitro, it can only be used under reducing conditions since it depends on one or two catalytic cysteines in the intein active site. This severely limits the scope of protein trans-splicing since it excludes proteins that are sensitive to reducing conditions, such as those containing critical disulfide bonds (i.e., antibodies).
Prof. Shmuel Pietrokovski and his team developed cysteine-less (CL) split inteins that modify proteins efficiently under native and physiological conditions1.
Inteins are protein domains that auto-catalyze protein-splicing of their flanking regions with a peptide bond. In protein trans-splicing, two parts of a split-intein can be used to ligate or cyclize polypeptides in a traceless manner. The reaction is robust, can be performed in-vivo or in-vitro, using biologically or chemically synthesized peptides or proteins. The sequence of only 2-3 flanking amino acids is constrained. As shown in the figure below, the N and C terminal intein fragments (IntN & IntC) associate and fold into the active domain, thereby linking the flanking sequences with a peptide bond. All previously known split inteins only function in reducing conditions due to their use of one or two catalytic cysteines. In this invention, novel cysteine-less split inteins are capable of robust trans-splicing at ambient temperatures and without the requirement of any chemical reducing or denaturation steps. This allows the preservation of disulfide bonds within the target protein. The use of this split intein was demonstrated for a full-length IgG, an Fc fragment of an IgG antibody, and two nanobodies as representatives of therapeutically relevant proteins. The reactions are high yielding (> 90%) at low to medium micromolar concentrations.
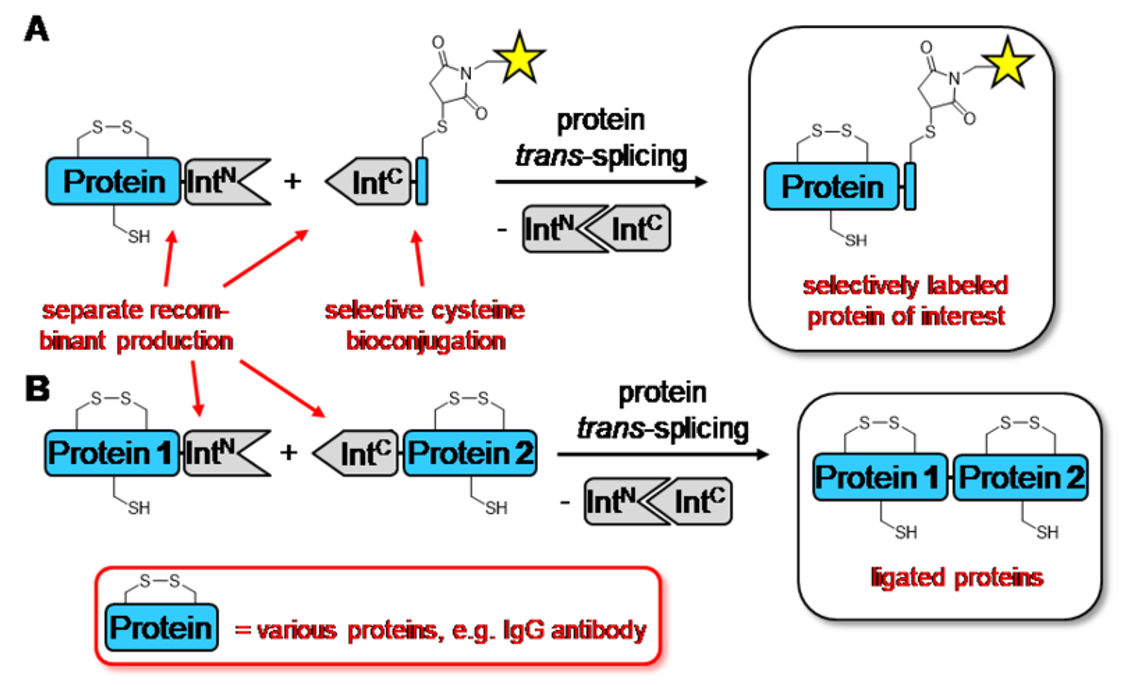
Figure 1 – Inteins mechanism of action
- Performed in the absence of reducing agents
- Reactions at ambient temperature; high yield
- Novel access to protein semi-synthesis and protein modification without denaturation
- Preservation of disulfide bonds and free cysteines
Bhagawati M, Terhorst TME, Füsser F, et al. A mesophilic cysteine-less split intein for protein trans -splicing applications under oxidizing conditions. Proc Natl Acad Sci. 2019;116(44):22164-22172. doi:10.1073/pnas.1909825116


