Chimeric Antigen Receptor (CAR)-T Cell Therapy is a personalized treatment for cancer in which the patient's T cells are genetically engineered to express a synthetic receptor that binds a tumor antigen. Although this treatment is very successful for treating some hematological cancers, it may cause a severe life-threatening side effect known as "Cytokine storm." The current technology enables the design of safer CAR-T cells with enhanced cytotoxicity and reduced cytokine release by modulating the transmembrane domain of the CAR.
CAR-T Cell Therapy is a cutting-edge immunotherapy modality with a global market that reached a value of nearly $734.0 million in 20191. In this method, T cells from the patient are isolated, activated, and engineered to express the CAR gene. This gene encodes a receptor designed to specifically recognize the patient's tumor and activate the T cells against it. The engineered CAR-T cells are expanded and transfused back to the patient to treat cancer. Despite the remarkable success of CAR-T cells in treating B cell leukemia and lymphoma, their therapeutic efficacy in solid tumors and hematological malignancies is limited and may cause severe life-threatening toxicities known as Cytokine Release Syndrome (CRS), or "Cytokine storm."2 Therefore, there is an unmet need to develop a modified CAR design to control the T cells' activation and tune their killing and cytokine production to make CAR-T cells safer without losing their efficacy.
The teams of Prof. Sarel Fleishman and his collaborators at the Walter and Eliza Hall Institute of Medical Research (WEHI) developed a computational platform for designing a new CAR with a unique transmembrane (TM) domain that forms specific oligomeric structures, which modulates the signaling in the CAR-T, resulting in reduced inflammatory cytokine release3.
The group of Prof. Sarel Fleishman developed a computational method for designing short (19-25 amino acids) segments that are inserted efficiently into biological membranes and form ultrastable complexes (homo-oligomers, i.e, dimers, trimers, and tetramers). Using this method, they designed new CARs with improved TM domains programmed to have specific oligomeric interactions. Their collaborators from WEHI showed that the CAR-T cells that expressed the newly designed CAR had superior cytotoxicity (Figure 1A) and attenuated inflammatory cytokine release (Figure 1B) compared to otherwise identical CARs containing natural TM domains. These results highlight the advantages of programming specific structural features in engineered receptors through de novo protein design for optimal CAR-T activity.
- Safer and efficient CAR-T cells, with high cytotoxic potency and reduced inflammatory cytokine release
- Improvements that can be integrated in existing CAR designs
- Synthetic receptor design for therapeutic applications
The group of Prof. Sarel Fleishman developed the computational platform for the de novo design of programmed membrane proteins (proMPs). They applied the method on CAR-T. To validate the method, the team programmed specific homotypic interactions in HER2-specific chimeric antigen without altering key functional domains in the extracellular and intracellular spaces. Their collaborators from WEHI showed that CAR-T cells expressing dimeric or trimeric proCARs exhibit enhanced target-cell cytotoxicity in vitro compared to a reference CAR similar to those currently used in the clinic. Additionally, all proCARs tested show strongly attenuated inflammatory cytokine release in vitro. In a mouse model of HER2+ cancer, anti‑tumor activity increased with oligomeric state, with the trimeric proCAR most closely approaching the efficacy of the reference CD28 TMD-containing CAR-T (Figure 1A). As next steps, the teams intend to test proCAR function in human T-cells in vitro and to design additional proCAR on other CAR backgrounds.
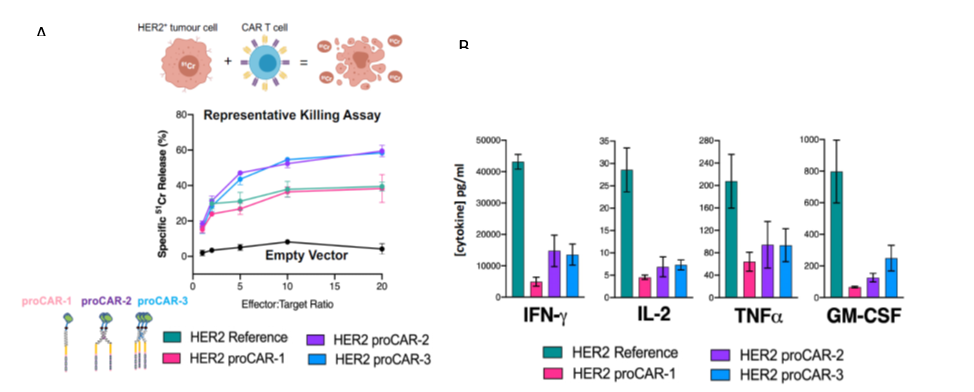
Figure 1 – (A) Assay measuring cytotoxic potency of HER2-directed primary mouse proCAR-T cells against human-HER2+MC57 mouse fibrosarcoma target cells. (B) Cytokine production by primary mouse HER2 proCAR-T cells co-cultured with MC57-HER2 target tumor cells.
Elazar A, Chandler NJ, Davey AS, et al. De Novo Designed Receptor Transmembrane Domains Enhance CAR-T Cell Cytotoxicity and Attenuate Cytokine Release. bioRxiv; 2020. doi:10.1101/2020.07.26.221598


