CRISPR-Cas9 is a powerful tool for genome editing, widely used for basic research and the development of treatments for genetic diseases. However, its efficiency is currently limited. The current technology is a new chimeric Cas9 fused to a domain that recruits DNA repair factors directly to the genomic site that is being edited, thus doubling the efficiency of ‘error-free’ genome editing. This novel and highly efficient chimeric version of the Cas9 endonuclease is thus particularly relevant for clinical gene therapy applications.
CRISPR-Cas9 is a powerful tool for genome editing that allows researchers to modify genomes easily, accurately, and efficiently. It is widely used in various applications in basic as well as translational research and holds great promise in the development of new treatments for genetic diseases. For these reasons, CRISPR-Cas9 has entitled its co-developers the Nobel Prize in Chemistry for 2020.
CRISPR-Cas9 utilizes Cas9, which makes a double-strand break in the genome in a specific location directed by a guide RNA. Once the cut is made, new DNA sequences can be introduced by employing the intrinsic DNA repair machinery, homology-directed repair (HDR). However, the DNA breaks can also be repaired without adding the desired DNA sequences by an alternative, more predominant cellular mechanism, called non-homologous end-joining (NHEJ). Therefore, achieving the desired result can often be inefficient. Previous approaches to this problem have used knockdown or pharmacological inhibition of NHEJ components, or cell cycle manipulation, in order to shift the balance towards HDR. However, altering the global DNA repair mechanism in the cell may result in undesired random mutations.
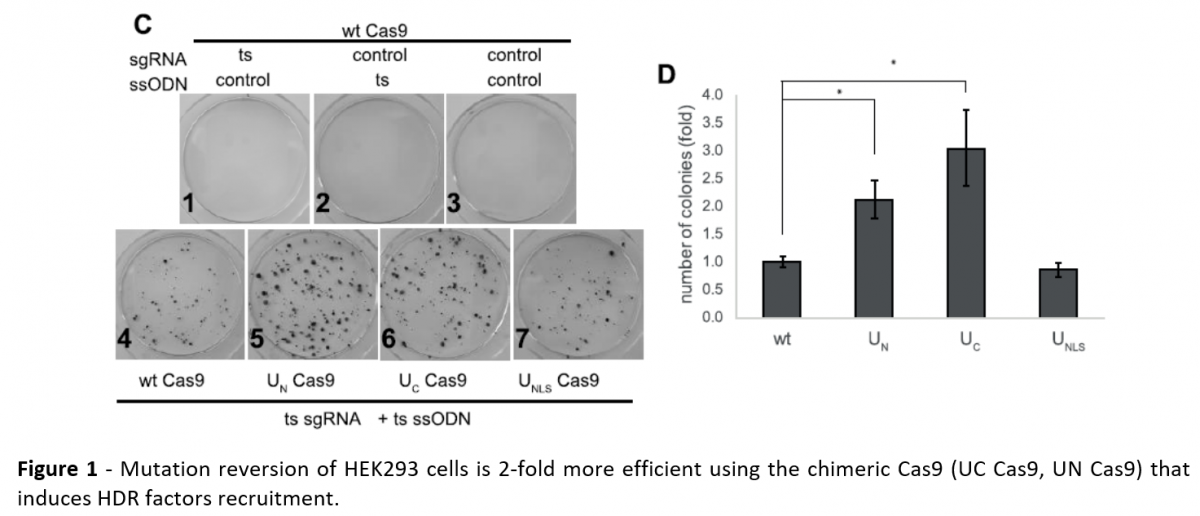
Yosef Shaul and his team developed a chimeric Cas9, which targets HDR factors directly to the DNA break site, resulting in a two-fold more efficient genome editing.
To increase the efficiency of CRISPR-Cas9 genome editing, HDR factors are targeted directly to the break site. This is achieved by a newly designed chimeric construct of Cas9, where the Cas9 nuclease is fused to a viral domain taken from Herpes Simplex Virus (HSV-1). This domain is a short, intrinsically disordered sequence that specifically recruits cellular factors that facilitate HDR. The chimeric Cas9 construct was two-fold more efficient in promoting specific editing of genomic loci and highly efficient in correcting a genomic point mutation. These chimeric Cas9 constructs provide a simple and versatile means for improving CRISPR-mediated genome editing.1,2
- Useful for gene therapy applications and basic science research
- A simple method that only requires substitution of the Cas9 protein with the modified version, and no other special reagents or treatments are required
- Short fused recruitment domain which lacks intrinsic enzymatic activity
- The chimeric Cas9 is more efficient at very low concentration of the protein, predicted to have fewer off-target effects, therefore it is particularly relevant for clinical applications
- Promoting HDR machinery locally at the break site without perturbing global DNA repair pathways
The chimeric protein was designed, purified and successfully tested on several human, mouse, and hamster cell lines, as well as in a clinically-relevant setup, namely by targeted editing of the SCD causative mutation at the HBB locus in human CD34+ hematopoietic stem and progenitor cells. The editing was made using small insertions/point mutations and large insertions (e.g. fluorescent proteins/other cassettes encoded by plasmid donors).
Benitez EK, Lomova Kaufman A, Cervantes L, et al. Global and Local Manipulation of DNA Repair Mechanisms to Alter Site-Specific Gene Editing Outcomes in Hematopoietic Stem Cells. Front Genome Ed. 2020;2:60154 doi:10.3389/fgeed.2020.601541
Reuven N, Adler J, Broennimann K, Myers N, Shaul Y. Recruitment of DNA Repair MRN Complex by Intrinsically Disordered Protein Domain Fused to Cas9 Improves Efficiency of CRISPR-Mediated Genome Editing. Biomolecules. 2019;9(10):584. doi:10.3390/biom9100584


