Autophagy plays a vital role in the survival of many solid tumors, often leading to drug resistance in chemotherapy and targeted treatments. Prof. Adi Kimchi's team has developed a high-throughput screening platform to identify small molecules that specifically inhibit the interaction of two crucial proteins in the autophagy machinery. Through an in vitro high-throughput screening on cell lysates and validation in cell-based assays and patient-derived cells, several hit compounds were identified and optimized. Specific autophagy inhibitors are highly desired as the current options in the market are not specific and have multiple off-target effects.
Tumors often rely on high levels of autophagy, a process known as "autophagy addiction," for survival. This includes tumors that are genetically dependent on autophagy for their growth due to mutations that require high metabolism and energy, as well as tumors that develop an addiction to autophagy as a result of exposure to stress during chemotherapy and targeted treatments. Autophagy is, therefore, a promising target for the development of new therapies for solid tumors and for preventing drug resistance. However, current autophagy inhibitors are not specific and can have off-target effects, making the development of specific autophagy inhibitors necessary.
Prof. Adi Kimchi and her team have developed a high-throughput screening platform to identify small molecules that specifically inhibit the interaction of two essential proteins in the autophagy machinery. By targeting autophagy, this technology has the potential to revolutionize the treatment of solid tumors and prevent acquired resistance to current therapies.
The team developed a unique screening assay using a luciferase-based complementation method to identify small molecules that inhibit the interaction of two key proteins, ATG3-ATG12, in the autophagy process. These proteins are critical in both the degradative and secretory arms of autophagy. Out of approximately 110,000 compounds screened, hits were identified at a rate of around 1.85%. These hits were re-synthesized and subjected to a dose-response assessment. Candidates with an IC50 of 10-40 µM (approximately 20 compounds) were further tested in a cell-based assay to confirm their inhibitory effect. Three of these compounds were selected for additional structure-activity relationship analysis, including the synthesis of derivatives. The two final hits were tested in patient-derived cancer cells with autophagy addiction (pancreatic and melanoma) and showed single agent and combination anti-cancer activity.
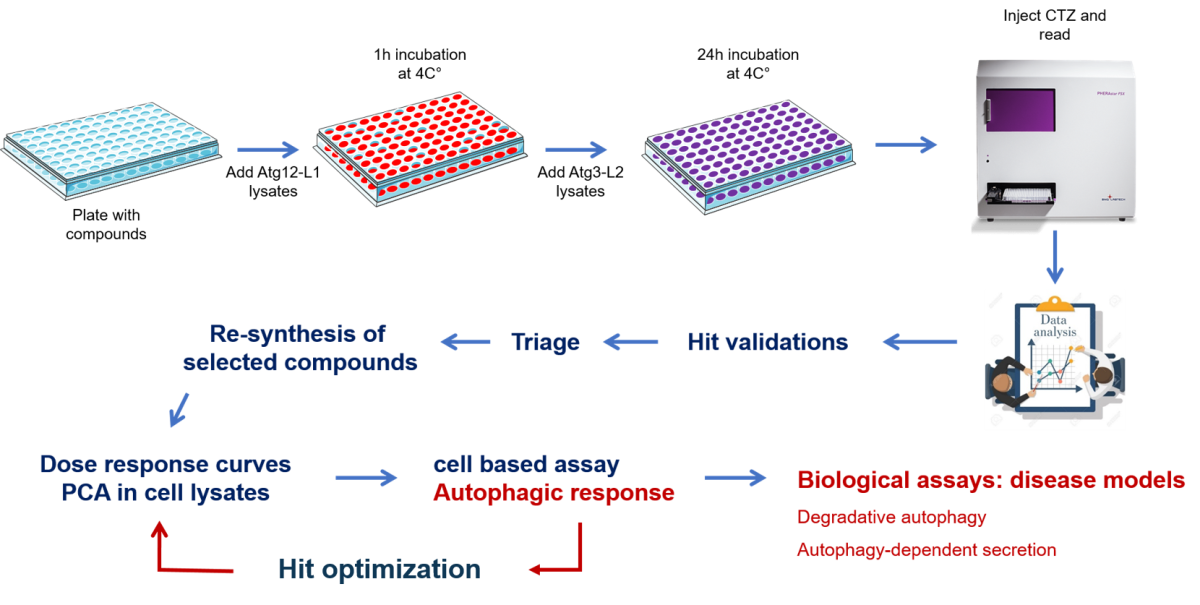
The screening platform assay has been thoroughly validated, including cell-based assays for hit validation. Three compounds identified as hits have been patented.

