Using affinity maturated TCRs to generate reactive T cells
Immunotherapy, that is the use of the immune system to treat cancer, is currently a leading candidate in the combat against cancer. Unlike the toxic effects of both chemotherapy and radiation, immunotherapy is considered to have mild side effects due to its ability to differentiate between healthy and cancerous cells. Also, the therapeutic role of the immune system is an essential element in the healing process due to bone marrow transplantation for hematologic malignancies.
However, a more efficacious and less toxic T cells based treatment is required. Effective therapy depends on the functional avidity between T cell receptors (TCRs) and peptide-MHC complex (pMHC). However, the natural affinity of anti-tumor TCRs is low and they do not naturally undergo the processes that improve antibody affinity, such as somatic hypermutation (SHM). Currently there are a number of methods for increasing the affinity of a TCR to its ligand, however each method has its limitations.
One approach is to isolate higher affinity TCRs from mice, to which the human antigen is foreign, with human MHC molecules. This approach has been successful, however, it is not robust. An alternative strategy to overcome T cell tolerance is based on the in-vitro affinity maturation of TCRs isolated from low avidity T cells. A number of methods have been developed. Most involve randomly mutating a small region, followed by selection on phage, yeast, or T cell display. These approaches have also yielded higher affinity TCRs, however there are limitations. These include that the mutations are only in a small region, for T cell display a small library size, and for yeast and phage the TCR must be modified to be expressed and some TCRs have had affinity so high that specificity is lost.
In the adaptive immune system repertoire diversity is achieved through somatic alterations of the immunoglobulin (Ig) locus. Somatic hypermutation (SHM) introduces point mutations into the V region antigen-binding pocket, creating Ig variants with enhanced affinity for a particular Ag. These mutations arise at a rate of 10−3/basepair/generation, several orders of magnitude above the rate of spontaneous mutation. Activation-Induced Cytidine Deaminase (AID) is the key factor that triggers SHM. Antibodies improve their affinities over time via SHM. However, TCRs do not undergo SHM.
This technology addresses three needs (1) a need for more broad and effective means for generating T cells that bear high avidity TCR that can recognize their MHC-peptide ligands on tumor cells and used in adoptive cell transfer (2) a need for a robust TCR affinity maturation system which can target all regions, including the constant region which can be applied to other TCRs (3) a need for a TCR affinity maturation system without strict limitations on library size of TCR variants
This technology presents a new method of increasing the affinity of a TCR to its ligand. This is done by subjecting TCR genes to SHM via the enzyme Activation Induced cytidine Deaminase (AID). The technology further provides affinity maturated TCRs (in cell- bound or in soluble form) and their pharmaceutical potential for immunotherapy.
First a nucleic acid construct encoding a TCR gene is expressed in a host cell. Next SHM is used to introduce mutations to the TCR gene. Last, the cells expressing the TCR with high affinity to the ligand are selected. Any TCR with a known DNA sequence and antigen can be used in this system.
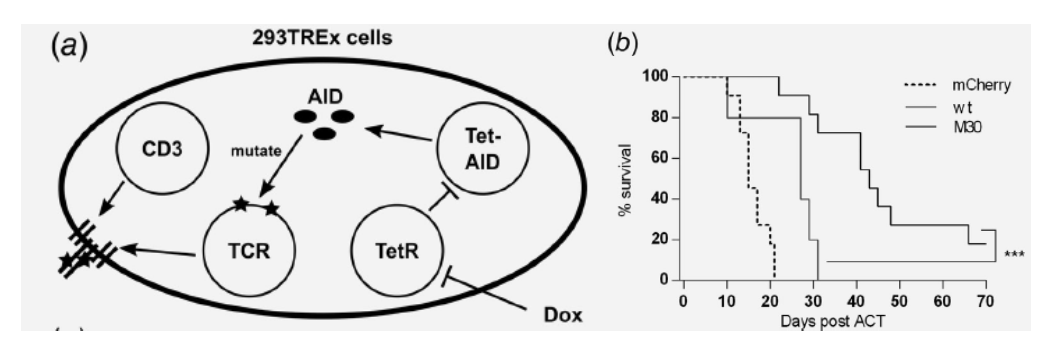
1: SHM-based TCR avidity maturation system design and improved antitumor response in-vivo. Schematic representation of SHM system in 293TREx cells (a). C57Bl/6 mice were inoculated with 2 × 105 mhgp100‐B16 tumor cells subcutaneously, irradiated (5 Gy) after 9 days, and adoptively transferred with 4 × 106 transduced T cells the next day. Survival was compared 70 days post ACT (log‐rank test, p = 0.0004) (Bassan, 2019).
- Generating anti-tumor T cells
- Generating T cells reactive against selected antigen
- Robust, effective
The approach was validated in cell lines, primary T cells and tested for efficacy in syngeneic mouse models. Ready for development towards clinical applications
Bassan, D. et al. ‘Optimizing T-cell receptor avidity with somatic hypermutation’, Int.J.Cancer. 2019 Nov 15;145(10):2816-2826. doi: 10.1002/ijc.32612. (2019)


