In recent years, complexes based on cooperating ligands have exhibited remarkable catalytic activity. These ligands can cooperate with the metal center by undergoing reversible structural
changes in the processes of substrate activation and product formation.
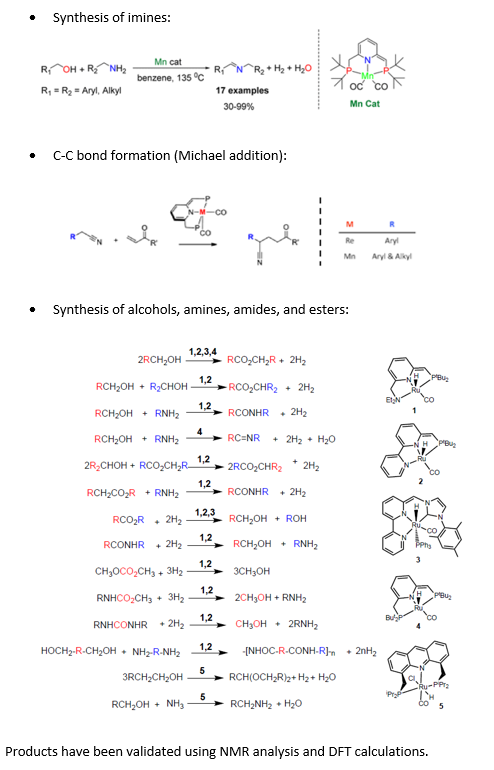
Prof. David Milstein's group has discovered a new mode of action for metal-ligand cooperation, involving aromatization, and dearomatization of ligands. Pincer-type, pyridine-based complexes of Mn
and acridine complexes of Ru have been shown to exhibit such cooperation, leading to facile activation of C-H, CC, H-H, N-H, and O-H bonds.
Amines, alcohols, amides, imines, and esters, are essential for industries such as research, chemicals, plastics and polymers, dyes, fragrances, fibers, pharmaceuticals, and agrochemicals. However, require conditions of high temperature/pressure and stoichiometric amounts of different hazardous compounds (e.g. activated acid derivatives or large amounts of metal compounds) to synthesize.
Carbon-carbon bond formation reactions are of fundamental importance in organic chemistry. Yet, they often require the application of strong bases, which may not be compatible with various functional groups and can lead to undesired side reactions.
In addition, there is a strong interest in the replacement of noble metal catalysts by more economical and environmentally friendly catalysts, based on earth-abundant metals. Further there is a need for cheaper and more efficient synthetic methods.
A novel set of catalysts based on manganese, ruthenium, and related borohydride complexes (Pincer-type) gives an alternative green option in synthesizing fundamental compounds.
In recent years, complexes based on “cooperating” ligands have exhibited remarkable catalytic activity. These ligands can cooperate with the metal center by undergoing reversible structural changes in the processes of substrate activation and product formation.
Prof. David Milstein’s group has discovered a new mode of action for metal-ligand cooperation, involving aromatization–dearomatization of ligands. Pincer-type, pyridine-based complexes of Mn and acridine complexes of Ru have been shown to exhibit such cooperation, leading to facile activation of C-H, C-C, H-H, N-H, and O-H bonds.
Advantages
- Reduced material cost (cheaper catalysts & reagents, reduced need for protecting groups, fewer reaction steps, higher yields, and less work-up.)
- Lower waste treatment cost (catalytic amounts - 1% mol, absence of toxic reagents, and byproducts, minimizes hazardous waste formation.)
- Decreased energy costs (Mild conditions - pressure 10 atm, temperatures of ~110 Co)
- No additives (just substrates and catalyst, except with certain reactions where hydrogen is required)
- New synthetic pathways that were not feasible before (e.g. such as the synthesis of amides and imines directly from alcohols and amines, esters synthesis from alcohols)
- Broad substrate scope and excellent yields
Applications
- Pharmaceuticals
- Dyes
- Cosmetics and fragrances
- Fibers
- Agrochemicals
The novel catalysts have been used in the following reactions:

Dr. Vered Pardo Yissar

