CRISPR-Cas9 is a widely-used tool for genome editing; however, it is very inefficient and often requires the addition of selection markers, forcing the introduction of foreign DNA elements. The current technology offers a strategy for generating an inherent host-derived selection gene by CRISPR technology simultaneously with the process of the targeted editing. The method is highly efficient and results in “scarless” gene editing.
CRISPR-Cas9 method, while promising, is currently inherently inefficient and requires selecting and enriching the edited cells. Current strategies involve the introduction of a second selectable editing event, allowing to enrich the population of edited cells (e.g., drug resistance). However, the selection is based on adding a foreign transgene, thus adding a genetic change on top of the desired change.
The team led by Prof. Yossi Shaul designed a CRISPR mediated genome editing method that eliminates the need for a foreign transgene making the selection "scarless” and efficient1.
To enrich for homology-directed repair (HDR)-dependent edited cells, the team employed a co-editing strategy, editing a gene of interest (GOI) concomitantly with rescuing an endogenous pre-made mutation. The rationale is that once the selectable gene is generated, the probability of the second concurrent hit is higher, and therefore an increased incidence of gene editing is obtained (Figure 1).
The team developed an inherent host-derived selection gene, which is based either on temperature-sensitivity or Cycloheximide sensitivity. In the first case, a temperature‑sensitive (ts) cell line harboring a point mutation in the TAF1 gene that encodes to the largest component of the basal transcription complex TFIID was established (HEK293 TAFts). To edit the GOI by CRISPR technology, two simultaneous editing events are performed, targeting the ts gene simultaneously with the GOI. Cells that are correctly edited for ts are able to grow at high temperature (39°C) creating colonies where 10-90% of them are positive for the GOI editing as well.
In the second case, a selectivity marker based on Cycloheximide (CHX) resistance is generated by introducing a single point mutation in the ribosomal protein RPL36A. CHX treatment kills all the unedited cells, and the cells that are edited form colonies, from which 10-50% are also positive for the GOI editing as well. This is considered semi-scarless since the cells carry a drug-resistant mutation.
Another strategy for a semi-scarless HDR editing is by using fatal siRNA directed against an essential gene (e.g. DNA polymerases, RNA polymerases, ribosome components, proteasome components etc.). Cells in which a silent mutation is successfully incorporated in the targeted essential gene will be resistant to the cell death/growth arrest imposed by the siRNA. These cells are also likely to successfully edit the GOI.
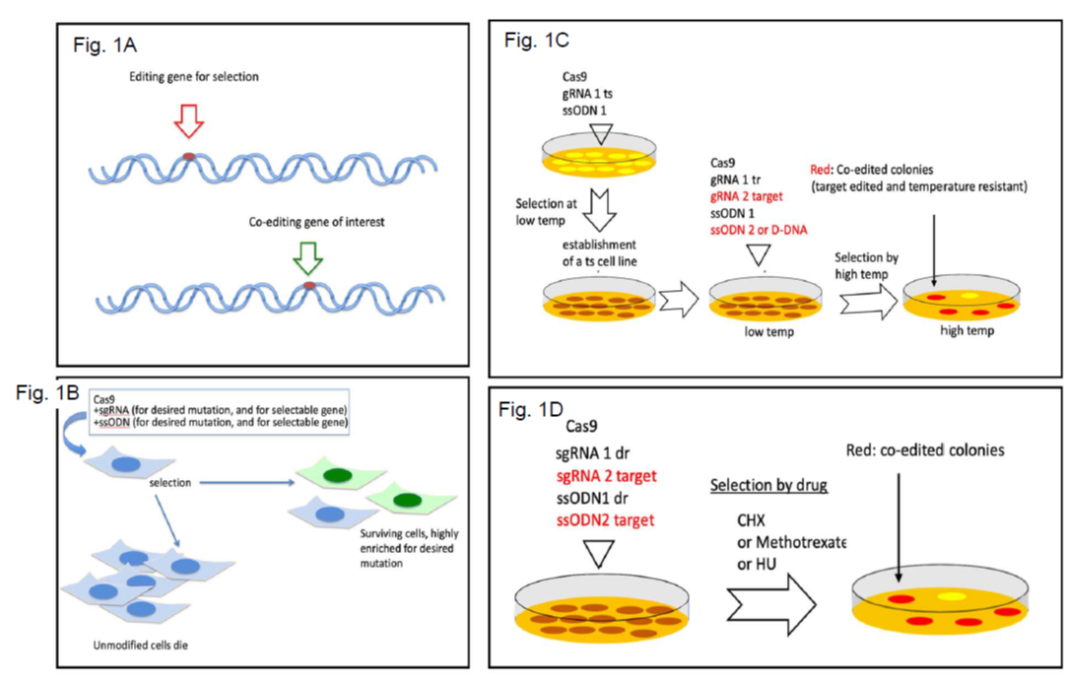
Figure 1 - Schematic presentation of the co-editing principle. A. HDR editing of a selectable endogenous gene increases the probability of a second editing event in the cell. B. The experimental steps of co-editing in cells bearing endogenous selectable gene. C. A general principle of generating an endogenous selectable gene for use in co-editing experiments. D. Using a drug resistant mutation (dr) for coediting.
- A method for Scarless DNA editing without introducing additional foreign DNA sequences
- Highly efficient – alleviates the need for single cell culturing employed by other methods
The team developed and fully characterized a temperature‑sensitive (ts) cell line, (HEK293 TAFts) that can be used as part of a kit for CRISPR based editing. In addition, they also developed a method for semi-scarless genome editing using drug selection based on an endogenous gene, which was successfully employed and fully characterized using Cycloheximide. Semi-scarless editing using siRNA strategy was also demonstrated.
Reuven N, Adler J, Myers N, Shaul Y. CRISPR Co-Editing Strategy for Scarless Homology-Directed Genome Editing. Int J Mol Sci. 2021;22(7):374 doi:10.3390/ijms22073741


