CRISPR-Cas9 is a powerful tool for genome editing, widely used for basic research and the development of treatments for genetic diseases. However, its efficiency is currently limited. The current technology is a new chimeric Cas9 fused to a domain that recruits cellular factors promoting homology-directed repair (HDR) directly to the edited genomic site, thereby doubling the efficiency of precise (‘error-free’) genome editing. This novel and highly efficient chimeric version of the Cas9 endonuclease is thus particularly relevant for clinical gene therapy applications.
- Gene therapy applications
- Basic science research
- Simple implementation – requires only the replacement of wild-type Cas9 with the modified version, with no additional reagents or treatments.
- The fused domain lacks intrinsic enzymatic activity, minimizing risk of off-target effects or interference with cellular processes
- More efficient at very low concentrations of the protein, predicted to have fewer off-target effects, therefore it is particularly relevant for clinical applications
- Promoting homology-directed repair machinery locally at the break site without perturbing global DNA repair pathways.
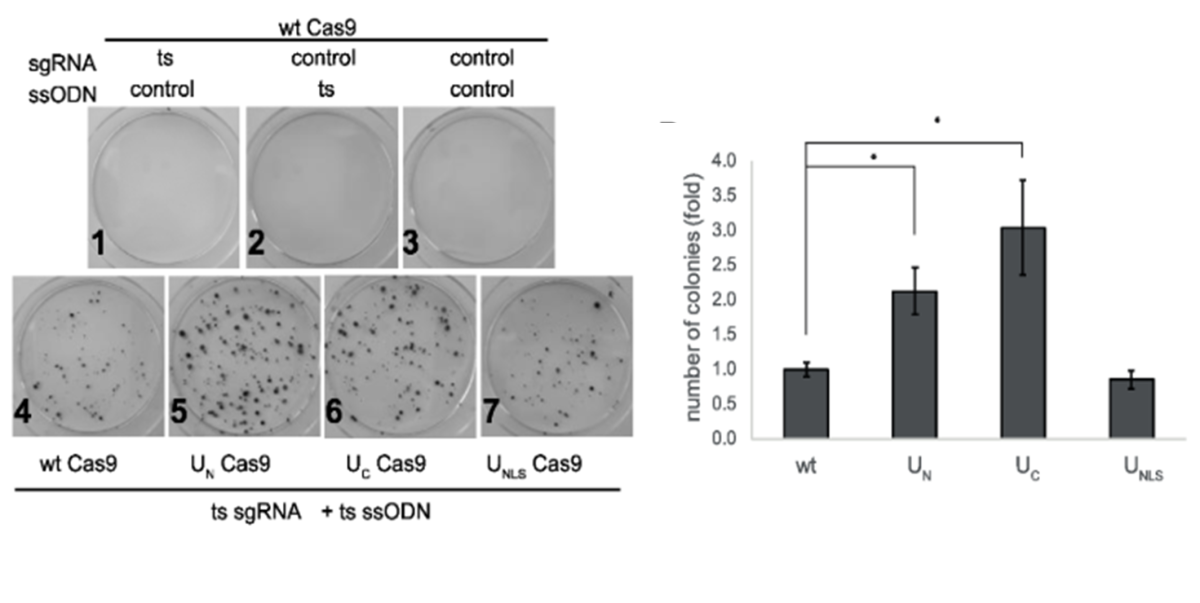
Mutation reversion of HEK293 cells is 2-fold more efficient using the chimeric Cas9 (UC Cas9, UN Cas9) that induces HDR factors recruitment.
The chimeric protein was designed, purified, and successfully tested in multiple human, mouse, and hamster cell lines, as well as in a clinically relevant setup—specifically, targeted correction of the SCD mutation at the HBB locus in human CD34+ hematopoietic stem and progenitor cells, using both small point mutations and large insertions (e.g., fluorescent proteins or other plasmid-encoded cassettes).


