Regulating ErbB2 signaling in cardiomyocytes (CM) as novel treatment for heart diseases by inducing regeneration of cardiac tissue.
Ischemic heart disease and heart failure are the leading causes of death in the western world. Although improved medical care reduced early mortality rate, survivors are susceptible to increased prevalence of chronic heart problems as they develop scarring and remodeling of cardiac tissue. Adult mammalian CMs have limited proliferative capacity with poor ability to replace lost tissue after acute ischemic injury. Currently, none of the existing treatments for heart failure is curative due to their disability to compensate the irreversible loss of functional CMs, besides heart transplantations which fail to suffice for all patients. Therefore, there is a major need for agents that can restore the proliferative capacity of CMs and regenerate the cardiac tissue.
The research team of Prof. Eldad Tzahor showed that ErbB2 is required for CM proliferation in the embryo and can induce proliferation in mature CMs.
The ligand-receptor signaling network consisting of neuregulin 1 (NRG1) and its tyrosine kinase receptors ErbB4, ErbB3 and ErbB2 plays critical roles during heart development. Prof. Tzahor's group studied the NRG1-ErbB2 axis and showed that ErbB2 is necessary and limiting for NRG1-induced CM proliferation in the neonate. Therefore, the group examined the possibility to use ErbB2 as a target for induced cell proliferation and regeneration in adult hearts. Their results show that ectopic induction of ErbB2 signaling in adult CMs ‘rejuvenates' them to an immature state, characterized by dedifferentiation and cell division. These were accompanied by profound sarcomere disassembly, reduction and replacement with immature components as well as by increased expression of CM dedifferentiation markers. In addition, it was shown that the downstream effectors ERK, AKT and GSK3β/β-catenin differentially mediate ErbB2-induced CM proliferation, dedifferentiation and hypertrophy, implicating them as possible targets as well. Thus, NRG1 treatment can be improved either by preventing the loss of ErbB2 in CMs or by targeting ErbB2-specific downstream signaling molecules. The present discovery strongly suggests that CM replacement by proliferation and dedifferentiation is a valuable strategy for regenerated myocardium.
- Induction of cardiomyocytes replacement as therapy for heart injury.
- Straightforward methodology which avoids complications associated with the requirement for cell transplantation
- Scheme of NRG1 and the ErbB2/4 heterodimer signaling cascades in MCs and their effect on growth, differentiation and proliferation.
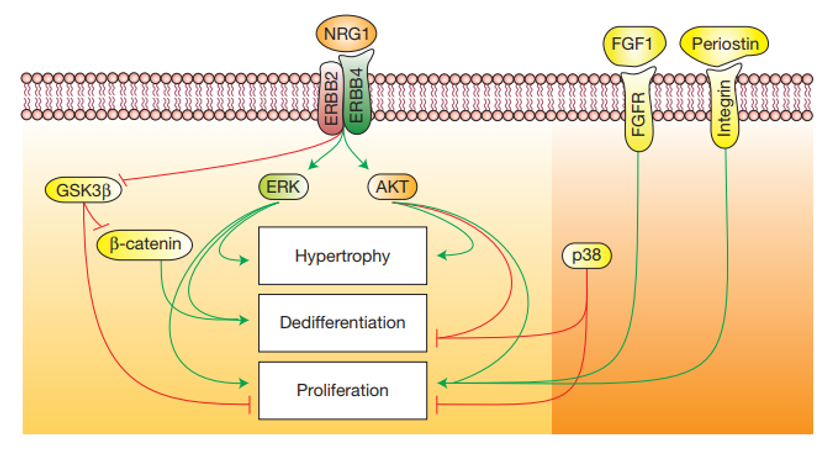
Prof. Tzahor's team used a variety of murine models, such as ErbB2 knock-out mice and constitutively-active ErbB2, to study the effect of ErbB2 on MC development, and demonstrated its role in neonates and adult mice. The team managed to induce heart regenesration in vivo by transient reactivation of ErbB2 signaling after myocardial infarction in a proof-of-concept experiment.
- ERBB2 drives YAP activation and EMT-like processes during cardiac regeneration. Nat Cell Biol 2020
- Single-cell analysis uncovers that metabolic reprogramming by ErbB2 signaling is essential for cardiomyocyte proliferation in the regenerating heart. Elife 2019
- The key roles of ERBB2 in cardiac regeneration. Cell Cycle 2015
- ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol 2015

