Sphingosine (SPH), a natural bactericidal agent which acts as a part of the human innate immune system in the skin, was found to be an effective treatment and prophylaxis for bacterial lung infections in cystic fibrosis (CF) mice. CF is the most common autosomal recessive disorder in western countries, affecting approximately 30,000 people in the US alone. A major risk in CF arises from chronic bacterial lung infections, affecting 80% of CF patients by the age of 25. The infections are currently treated with antibiotics, which rapidly become inefficient as resistant bacteria strains arise. The new technology is based on the discovery that both CF human patients and CF mice display reduced rates of SPH in the airways. Moreover, normalizing SPH levels by inhalation prevents or cures the infections in CF mice, thus rendering SPH a potent therapeutic agent for CF patients, an alternative to antibiotics.
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene and is the most common autosomal recessive disorder in western countries. Among other disease-related symptoms, lung symptoms determine the quality of life and life expectancy of most CF patients, with patients displaying high susceptibility to lung infection with Pseudomonas aeruginosa. Approximately 80% of CF patients suffer from chronic P. aeruginosa pneumonia by the age of 25. Pulmonary P. aeruginosa infections are also of major clinical importance in patients with chronic obstructive pulmonary disease (COPD), trauma, burn wounds, sepsis, or in patients requiring ventilation. At present, physiological innate defense mechanisms against this pathogen and their alterations in lung diseases remain unknown and therefore, lung infections are primarily treated with antibiotics. However, the increasing rate of P. aeruginosa resistance to many antibiotics necessitates the development of alternative therapies for the prevention and treatment of pulmonary bacterial infections.
The proposed technology suggests a novel therapeutic approach for the prevention and treatment of bacterial lung infection in susceptible populations, especially CF patients, by reinforcing the innate immune system with inhalation of SPH or SPH analogs.
Tech essence Sphingosine (SPH) is known to act as a bactericidal agent that protects human skin from bacterial colonization. SPH is generated by enzymatic hydrolysis of ceramide by acid ceramidase (AC). In a recent study, prof. Futerman and his team have found that SPH acts as a natural antibacterial agent in airways to prevent bacterial lung infection in healthy individuals. They have shown that in epithelial lung cells from CF patients and CF mice SPH levels are significantly reduced due to reduced AC activity and that inhalation of CF mice with SPH, AC or FTY720 (a SPH analog in clinical use for treating multiple sclerosis) normalizes SPH levels. Importantly, they demonstrated that inhalation with SPH, AC or FTY720 one hour after P. aeruginosa infection cures CF mice from the infection. Moreover, inhalation one hour prior to P. aeruginosa infection protects CF mice from infection. Next, the Futerman team in Weizmann Institute has shown that SPH has a broad anti-bacterial activity independent of immune cells activity. It directly kills a broad spectrum of pathogenic bacteria at nanomolar to low micromolar concentrations, including Pseudomomas aeruginosa, Acinetobacter baumannii, Haemophilus influenza and Moraxella catarrhalis. In summary, the new discovery strongly indicates that patients susceptible to bacterial lung infection could be treated by administration of SPH or SPH analogs.
- A novel therapeutic approach to prevent or cure bacterial lung infection.
- The new therapy is based on reinforcement of the physiological innate immunity rather than on antibiotics.
- The new therapy can be easily administered, via inhalation.
- FTY720, a SPH analog, is already in clinical use for treating multiple sclerosis
- Alternative treatment for bacterial lung infections.
- A prophylaxis for patients susceptible to bacterial lung infections.
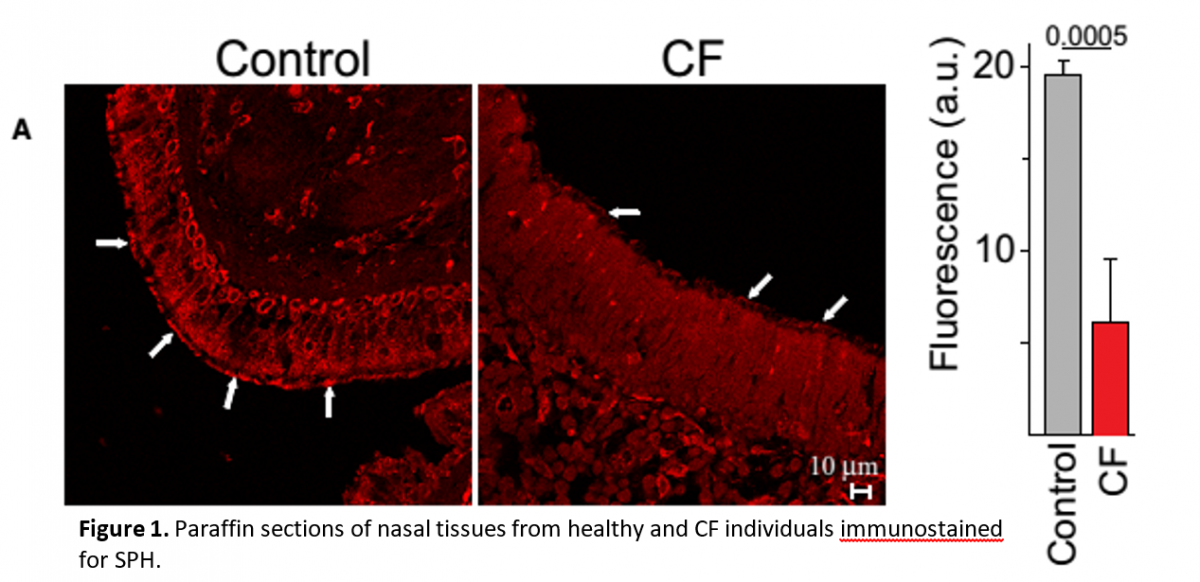
Reduced SPH levels in nasal tissues of CF human patients compared to healthy subjects.
Reduced SPH levels in trachi of CF mice can be rescued by inhalation of AC or SPH.
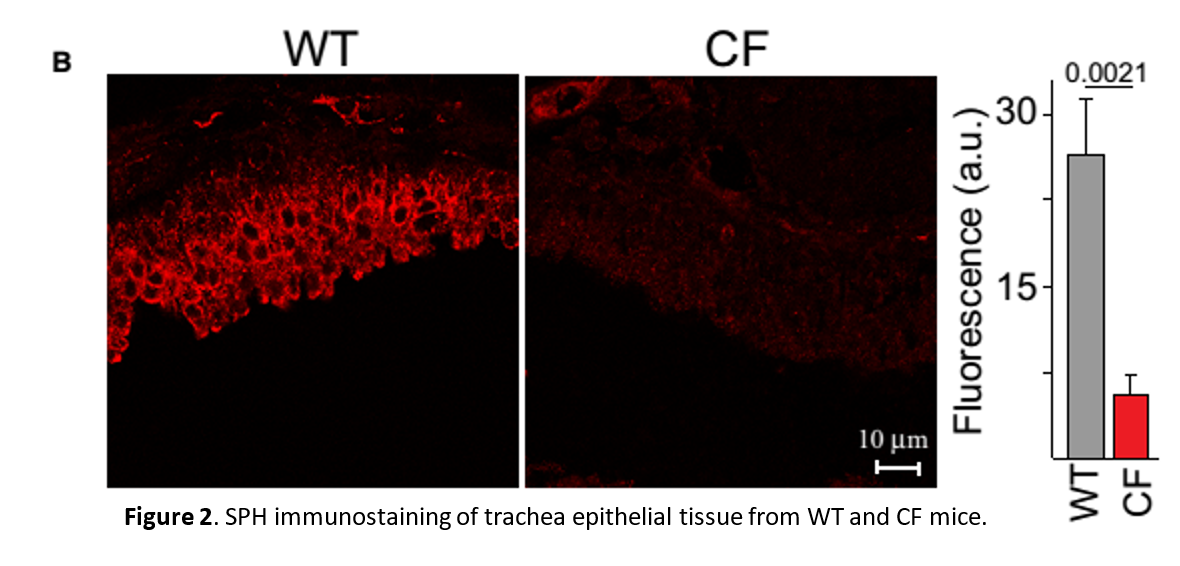
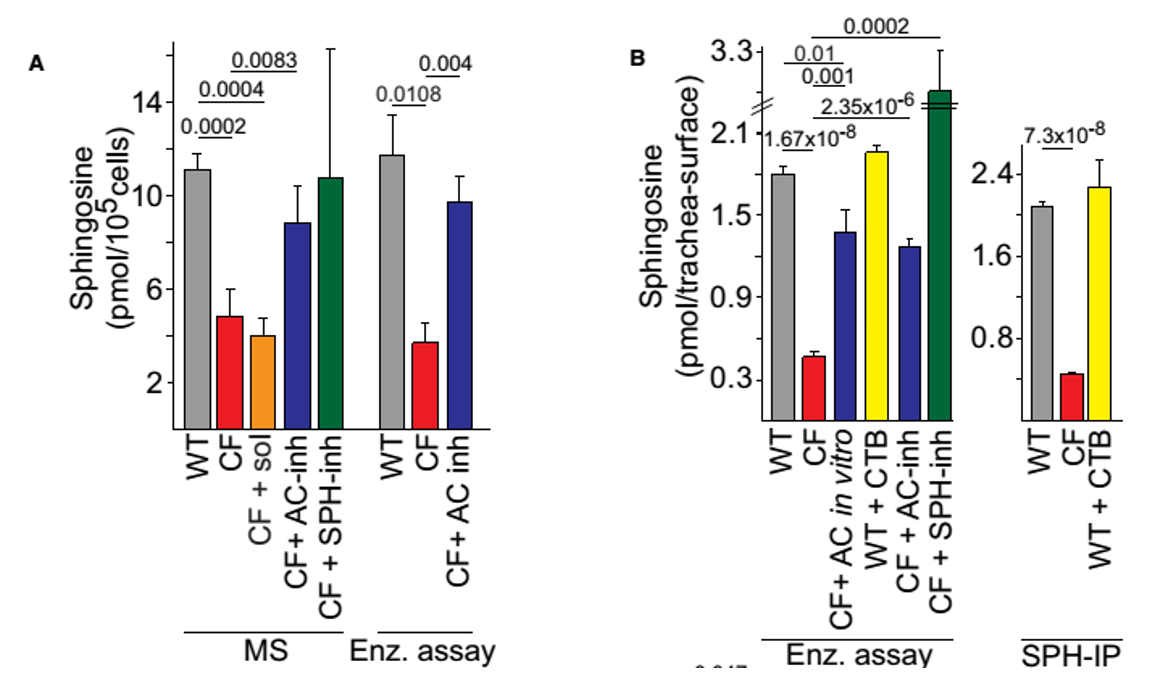
Figure 3. Biochemical analysis of SPH levels. (A) SPH levels were determined by mass spectrometry (MS) or an enzymatic kinase assay (Enz. assay) in lysates from isolated tracheal epithelial cells or (B) by an in situ enzymatic kinase assay on the tracheal surface (left) or by immunoprecipitation of SPH (SPH-IP) from the luminal surface followed by quantification using an enzymatic assay (right). Pre-incubation of WT trachea with cytochalasin B (CTB) did not change SPH surface levels. Incubation of trachea in vitro with AC proved the specificity of the enzymatic assay. Inhalation (inh) of AC (200units) or SPH normalized total SPH levels in isolated tracheal epithelial cells (A) and on the luminal surface (B), the solvent (sol) was without effect.
Inhalation of AC, SPH or SPH analog prior to or following infection with P. aeruginosa prevents or cures the infection, respectively.
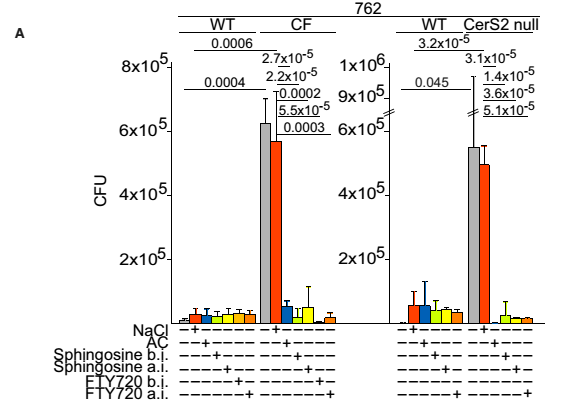
Figure 4. Number of bacteria in the lungs after intranasal infection with 1×108 CFU of P. aeruginosa strains 762. Mice were inhaled with 0.9% NaCl or AC 1h prior to infection (before infection, b.i.), or with SPH or FTY720 1h before or1h after infection (a.i.). Data are means±s.d., n=4. Numbers above bars indicate the exact calculated P-values.
SPH and other sphingoid LCBs are capable of directly killing P. aeruginosa in nanomolar to low micromolar concentrations.
Cystic fibrosis is the most common life-limiting autosomal recessive disease among people of European heritage. In the United States, approximately 30,000 individuals have CF and 1 in 3,500 children are born with CF. The mean annual health care cost for treating CF is US $15,571 per patient. Lifetime health care costs are approximately US $306,332 per patient. The majority of costs are accounted for by hospital inpatients (58%), followed by pharmaceuticals (29%), medical services (10%), complications (2%), and diagnostic tests (1%).
Pewzner-Jung, Yael, Shaghayegh Tavakoli Tabazavareh, Heike Grassmé, Katrin Anne Becker, Lukasz Japtok, Jörg Steinmann, Tammar Joseph, et al. 2014. “Sphingoid Long Chain Bases Prevent Lung Infection by Pseudomonas Aeruginosa.” EMBO Molecular Medicine 6 (9): 1205–14.


