The production of alcohols, amines, amides, imines, and esters is central to pharmaceuticals, polymers, dyes, fragrances, and agrochemicals, but conventional syntheses require harsh conditions, toxic reagents, and expensive noble metals. This technology introduces a new class of pincer-type catalysts based on manganese, ruthenium, and related borohydride complexes. These catalysts operate via a unique aromatization–dearomatization mechanism, enabling efficient bond activation (C–H, C–C, H–H, N–H, O–H) and offering a green, economical alternative for essential organic transformations
- Synthesis of amines, alcohols, amides, imines, and esters for various markets for various markets:
- Cosmetics and fragrances (i.e. Ceramides and Acrylamide-based Polymers)
- Pharmaceuticals (i.e. Atorvastatin, Levetiracetam, Lidocaine)
- Dyes (i.e. Fluorescent Brighteners and Reactive Dyes)
- Agrochemicals (i.e. Propanil and Metalaxyl)
- Reduced material cost
- No additives
- Excellent yields
- Eco-Friendly - No waste treatment cost
- New synthetic pathways that were not feasible before
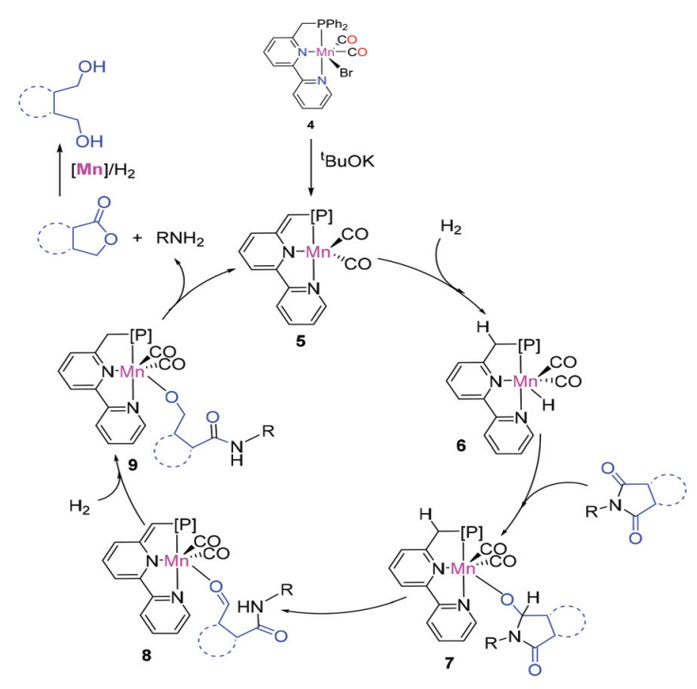
Proposed mechanism for the hydrogenation of cyclic imides
The catalysts have been successfully applied to key chemical reactions, including the synthesis of imines, alcohols, amines, esters, and C-C bond formation (Michael addition) on a lab scale.

Dr. Vered Pardo Yissar

