Covalent drugs have emerged as a groundbreaking modality for improving the affinity, potency, and duration of action of molecularly targeted inhibitors. Prof. London and his team have developed an innovative method for creating covalent biotherapeutics that can selectively react with both lysine and cysteine side chains extracellularly. This pioneering approach offers a scalable solution for generating covalent inhibitors with enhanced selectivity and potency, potentially unlocking new therapeutic targets and advancing the development of highly effective treatments for various diseases.
It is difficult to target certain therapeutic targets, such as transcription factors and protein-protein interaction interfaces, with traditional small molecules due to their complex binding surfaces. The combination of covalent electrophiles with peptide-based scaffolds combines the benefits of potency, selectivity and pharmacodynamics that arise from irreversible covalent binding with the ability of peptide binders to interact with extended, shallow protein surfaces. However, the current repertoire is primarily aimed at cysteine, which undergoes oxidation in the extracellular environment, leading to a limited range of available agents. Therefore, there is a critical need to expand the repertoire of covalent peptide and protein reagents that can engage with extracellular targets.
Introducing a robust platform for developing covalent biologics tailored specifically for extracellular targets.
The pipeline utilizes computational modeling to identify optimal candidates from known binding partners of a given target. Thioether-modified methacrylate electrophiles, offering a diverse reactivity profile, can be readily incorporated by direct, selective modification of the cysteine side chain. This enables the synthesis of binders from unprotected peptides or recombinant proteins. The resulting electophile reacts with lysine or cysteine side chains proximal to the binding site, forming covalent adducts (Figure 1).
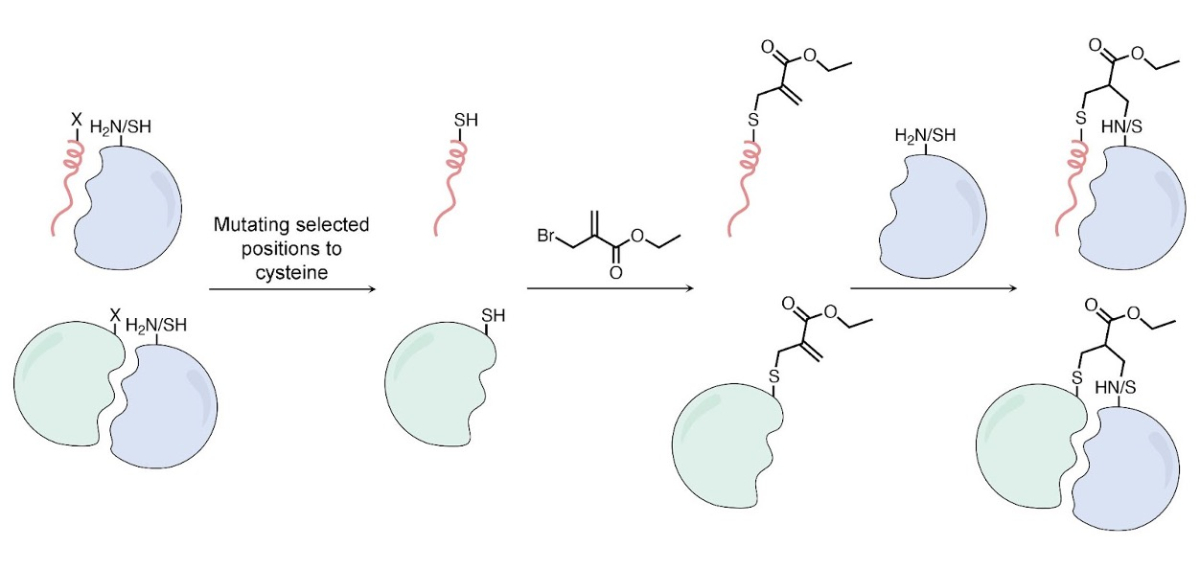
Figure 1. Scheme for generating thioether-based methacrylate covalent protein binders. A cysteine residue can be introduced into peptides (top) or proteins (bottom).
- Simplified and amenable to industrial scale-up (no requirement for non-canonical amino acids)
- Enables targeting diverse and previously undruggable theraptuic targets
- Well suited for clinical and research applications
The developed binders were shown to be effective and selective with their cognate targets in extracellularly secreted labeled proteins, as well as in cell lysates. Furthermore, computational model predictions have been successfully validated.
Gabizon R, Tivon B, Reddi RN, van den Oetelaar MCM, Amartely H, Cossar PJ, Ottmann C, London N. A simple method for developing lysine-targeted covalent protein reagents. Nat Commun. 2023 Dec 1;14(1):7933. doi: 10.1038/s41467-023-42632-5.

