The demand for innovative biofuel production methods remains a pressing challenge. Lignin, a major biomass component, poses a formidable obstacle since it is difficult to depolymerize and convert into biofuels. Natural enzymes known as Versatile Peroxidases (VPs), produced by white-rot fungi, exhibit efficient lignin depolymerization capabilities. However, the complexity of their structure hampers their production at industrial scales. Prof. Fleishman's team harnessed computational methods to design VPs with high stability and activity which are efficiently expressed in yeast cells. These engineered VPs hold immense potential for diverse industrial applications, ranging from biofuel production to bioremediation, biomedical uses, and even paper and textile processing.
Efficiently converting biomass into biofuels is a pivotal aspect of sustainable and renewable energy production. Lignin, the second most abundant biopolymer on Earth after cellulose, presents a prime candidate for biofuel conversion. However, lignin's amorphous and highly cross-linked structure obstructs the accessibility of chemicals and enzymes to degrade it, thus impeding its conversion into biofuels and other high-value chemicals. VPs are a group of natural fungal enzymes that efficiently depolymerize lignin. However, their intricate structure makes heterologous expression challenging, limiting their potential for research and industrial applications.
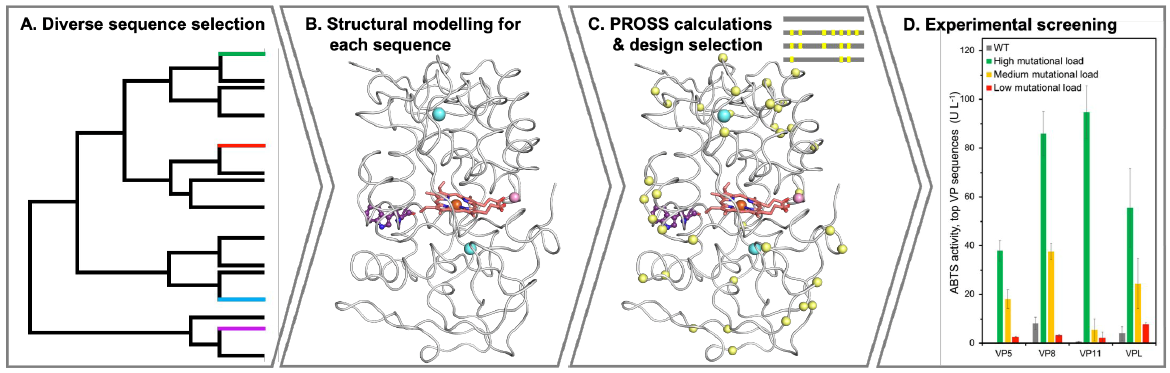
Utilizing advanced computational engineering methods, researchers at the Weizmann Institute developed a series of recombinant VPs with high stability and activity, which can be efficiently expressed in yeast cells.
Prof. Fleishman and his team leveraged computational design methods to engineer an optimized set of VPs, surpassing the stability and activity of their natural counterparts. The designed VPs exhibited high yeast expression levels, and each enzyme exhibited different substrate selectivity. Given the high heterogeneity of natural lignin, these enzymes can be used, as in fungi, in an efficient enzyme consortium for lignin degradation.
- High yield when expressed in yeast cells
- Improved stability
- Diverse substrate specificity
- A broad set of industrial applications, including biofuel production, bioremediation, textile industry, bleaching
The team designed 36 VPs, harboring as many as 43 mutations relative to the wild-type enzymes. Four of the designs were shown to be functionally expressed in yeast. The enzymes' stability under varying thermal and pH conditions were analyzed, alongside characterization of their kinetic parameters against five different substrates with varying redox potentials and chemical structures. Notably, three of these designs exhibit significant diversity in reactivity profiles and tolerance to environmental conditions, underpinning their potential for a wide range of applications.
Barber-Zucker S, Mindel V, Garcia-Ruiz E, Weinstein JJ, Alcalde M, Fleishman SJ. Stable and Functionally Diverse Versatile Peroxidases Designed Directly from Sequences. J Am Chem Soc. 2022;144(8):3564-3571. doi:10.1021/jacs.1c12433


