The coupling of transition-metal to photoredox catalytic cycles through single-electron transfer steps has become a powerful tool in the development of catalytic processes. The use of alternating current (AC), in turn, allows the redox processes related to one metal center to occur periodically at the same electrode resulting in a number of significant beneficial features. We provide, for the first time, a method to couple alternating current electrolysis to transition-metal catalysis through periodical electron transfer events. AC assistance facilitates catalytic reactions which involve reductive elimination or oxidative addition steps and shows higher yields and selectivity over the direct current (DC) approach.
Transition-metal catalysis is one of the most common forms of catalysis. However, the number of possible transition-metal-catalyzed reactions is fundamentally limited by the energetics and kinetics of catalytic cycles. The number of possible catalytic cycles could be greatly expanded by supplementing catalytic cycles with energy from light or electricity. Synthetic electrochemistry uses direct current almost exclusively. Reports on the coupling of direct current with transition metal catalysis that do not involve electrochemical oxidation or reduction of substrates are rare. To the best of our knowledge, there are no reports of AC-assisted transition metal catalysis in the literature.
Dr. Sergey N. Semenov and his team developed a new method for catalytic reactions based on the coupling of alternating current to transition-metal catalysis.
The coupling of electricity to transition-catalysis does not involve net oxidation or reduction of substrates but only reversible oxidation/reduction of a catalyst; thus, it does not require the use of DC. Dr. Sergey N. Semenov and his team demonstrated AC-enabled nickel-catalyzed amination, etherification, and esterification of aromatic halides1. The team achieved the successful couplings of a wide range of aryl bromides with primary and secondary alkyl amines and a variety of carboxylic acids and alcohols. The reactions were performed in the electrochemical cell, equipped with two glassy carbon rod electrodes, while NiBr2·DME (DME – dimethoxyethane) complex with bipyridine-type ligands was found to be highly efficient in the catalytic transformations and stable enough towards reversible oxidations and reductions. A schematic representation of the used setup and the results achieved for the AC-enabled nickel-catalyzed couplings are shown in Figure 1.
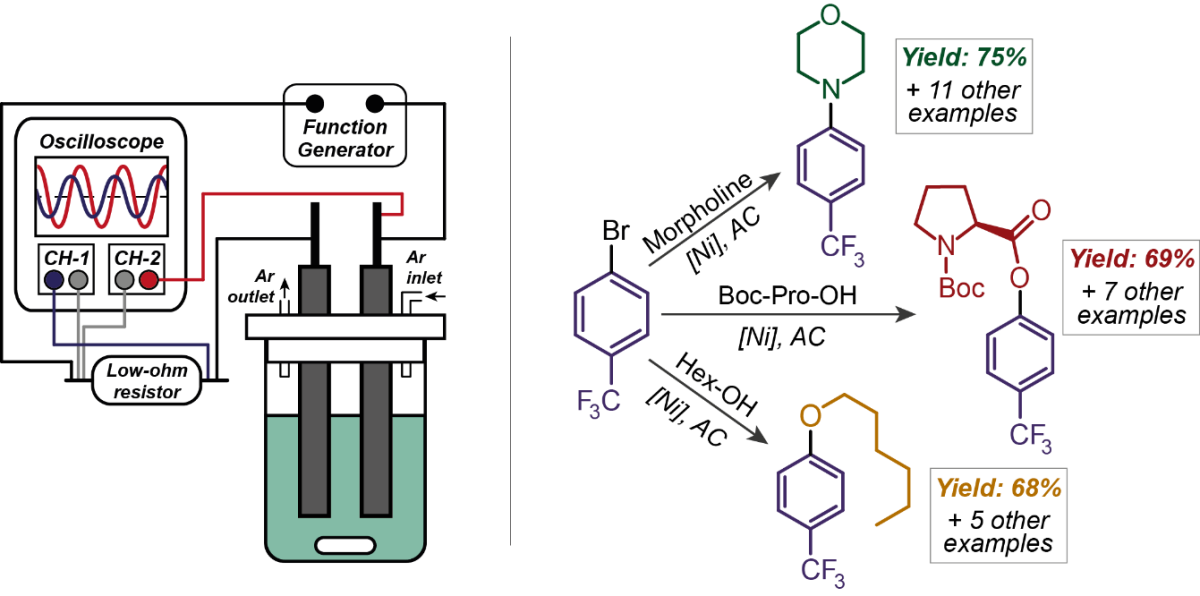
Figure 1: Demonstrated results of AC-enabled nickel-catalyzed cross-coupling reactions and used setup.1
Applications
- Catalytic systems where one or several steps can be accelerated by electrochemical oxidation/reduction
- Transition-metal catalytic reactions such as cross-coupling reactions, hydrogenation reactions, and hydroformylation reactions
Advantages
- Oxidation and reduction occur on the same electrode; therefore, reactions that require a short time interval between reduction and oxidation can be performed efficiently.
- The frequency and the waveform of AC are easily tunable experimental parameters that can be used to adjust the selectivity of reactions.
- AC may allow achieving the potentials beyond the solvent electrochemical window.
- AC allows the implementation of the concept of a stirring-free electrochemical reactor uniformly packed with mesh or porous electrodes.
The group proved the coupling of AC to transition-metal catalysis using three examples of related nickel-catalyzed coupling reactions (i.e., amination, etherification, esterification). The following steps of testing other catalytic systems are currently in process: (1) AC-assisted nickel-catalyzed synthesis of acrylic acid from CO2 and ethylene, (2) AC-assisted copper-catalyzed decarboxylative trifluoromethylation of carboxylic acids.
Catalysis is a key element in creating a sustainable chemical industry. The majority of bulk chemicals, as well as many fine chemicals and pharmaceuticals, are made via catalytic processes. AC-activated reactions can be used for:
Pharmaceuticals: The demonstrated catalytic reactions involve the formation of bonds that are commonly found in pharmaceutical compounds. Examples of relevant pharmaceuticals include Mepyramine, Antrafenine, Tetracaine, Leflunomide, Fluoxetine, and Crisaborole.
Synthesis of fine chemicals: The synthesis of acrylic acid from carbon dioxide (CO2) and ethylene in a nickel-catalyzed coupling reaction is another example of a reaction that has commercial potential. While acrylic acid is a widely-used valuable bulk chemical, atmospheric CO2 capture represents an increasingly relevant challenge of modern science.
- Bortnikov E.O, Semenov S.N, Coupling of Alternating Current to Transition-Metal Catalysis: Examples of Nickel-Catalyzed Cross-Coupling. The Journal of Organic Chemistry 2021 86 (1), 782-793. https://doi.org/10.1021/acs.joc.0c02350

Dr. Vered Pardo Yissar

