Nanostructured silica is a promising material with remarkable properties, such as high porosity, biocompatibility, and stability. The combination of these properties makes nanostructured silica highly applicable for catalysis, substances encapsulation, drug delivery, bioimaging, biosensing, energy storage, etc. Although these nanomaterials are being synthesized in nature by microorganisms (such as diatoms), current artificial synthesis strategies use harsh chemical conditions, are expensive, and not environmentally friendly.
Dr. Assaf Gal and his coworkers developed a green biosynthesis of nanostructured silica that mimics a natural process using positively and negatively charged polymers as a growing substrate for the silica. This newly developed synthesis enables the formation of nano and micro-scale silica structures and controls their morphologies, composition, and porosity. The naturally inspired process redundant the use of organic silicone precursors (Si(OR)4) and organic solvents, and substantially reduces the energy requirements for preparing nanostructured silica.
Nanostructured silica is a group of materials composed of silica with a controllable, homogeneous, and functionalized nanosurface. The combination of a large and controllable surface area, with the stability and biocompatibility of the silica skeleton, makes nanostructured silica very useful for various applications, including catalysis, substances encapsulation, biosensing, drug delivery and paints. However, their synthesis is limited today by using organic silicone precursors, organic solvents, harsh basic conditions, and high energy requirements. Developing a synthetic procedure for nanostructured silica synthesis in water using non-organic precursors will decrease costs and accelerate its use.
It was discovered that polyanion-polycations phase separation could initiate the growth of controlled silica structures from Si(OH)4 solution. The use of polyacrylic acid (PAA)- polyanion and polyallylamine hydrochloride (PAH)-polycation abled the silicification out of an aqua solution of Si(OH)4.
Silicification is a process where silicone precursor is polymerized into a dense SiO2. Usually, when Si(OH)4 is allowed to polymerize in an aqueous solution, it forms a useless low-density silica gel. The highly dense interface between the phase-separated polyanion and polycation polymers stabilizes the silicification by the polymers' charges and forces the mineral's growth. Adjustment of the reaction conditions, such as ionic strength, polymer composition, and silicon concentration, is used to control the nanostructured silica size, morphologies, porosity, and composition. A variety of structures can be synthesized, such as spheres, wires, and networks. Porous forms of silica can also be tuned in their density and porosity, allowing for the potential encapsulation and control release of active substances.
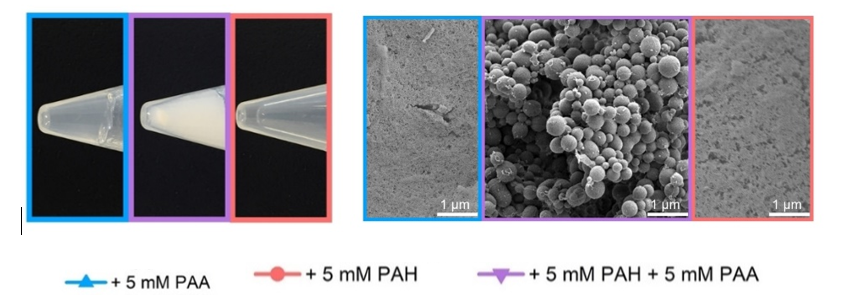
Figure 1- Silicification of Si(OH)4 in aqueous solution with polyanion (blue), polycation (red), and both polyanion and polycation (purple)
Applications:
- Catalysis
- Drug delivery
- Biosensing
- Bioimaging
- Food industry
- Paints
- Substances encapsulation
- Scaffold for tissue engineering
Advantages:
- Green chemistry
- Inexpensive
- Biocompatible
- Highly stable material
- Tailored morphology and sizes
- Controlled porosity
- Easily modified surfaces
Varied morphologies of nanostructured silica were synthesized consistently in small scale from Si(OH)4. Applicative uses are still in development and need further research.
Nanostructured silica could be applied to various industries: foods, medicine, cosmetics, paints, and catalysis. Simple production process using only biocompatible materials, without harsh toxic reaction agents, makes it more suitable and practical for many uses. Enabling the synthesis without the need for expansive precursors (Si(OH)4 is much cheaper than Si(OR)4), and pricey solvents (water vs. organic solvents) are estimated to reduce the production cost of these superior materials dramatically.
[1] H. Zhai, T. Bendikov, A. Gal, Angew. Chem. Int. Ed. 2022, 61, e202115930; Angew. Chem. 2022, 134, e202115930.H.
doi.org/10.1002/anie.202115930

Dr. Vered Pardo Yissar

