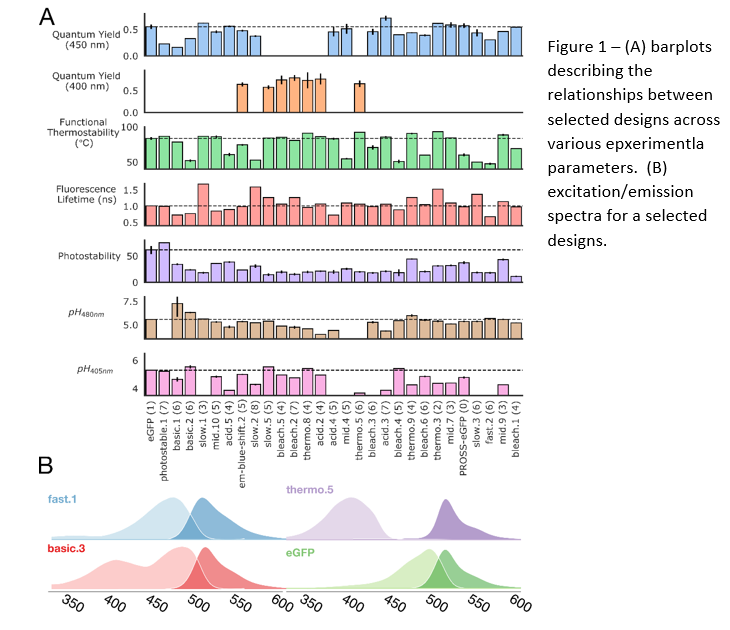
A collection of GFP variants with improved/unique properties, such as increased thermostability, photostability, quantum yield, altered fluorescence lifetime, pH sensitivity, and excitation spectra.
The team led by Prof. Fleishman developed a machine-learning-based approach called htFuncLib to design stable mutations in proteins. They applied htFuncLib to the chromophore-binding pocket of eGFP, synthesized 11 million designs in E. coli, and isolated 16,000 unique fluorescent designs encoding up to 12 active-site mutations relative to eGFP. Among the designs, many exhibit large and useful diversity in thermostability, photostability, quantum yield, fluorescence lifetime, pH sensitivity, and excitation spectra.
The collection offers a variety of stable GFP mutants, from which the optimal GFP can be selected based on the experimental needs, for example, thermostability (including GFP variants active in various temperatures, over 90°c), altered fluorescence lifetime, and altered excitation spectra.